Research Administration Portal (RAP)
- Why isn't CITI training showing up for an investigator?
- Can I have a lab assistant create a submission?
- Can I enter more than one principal investigator?
- Where should I be uploading forms and attachments such as the application, research plan, consent form, instruments, etc.?
- I am unable to locate my department, funding agency, research location, etc. from the drop-down lists. Do you have any tips to help search?
- I’m conducting on-line research. How do I answer the questions of which campus research locations the research activities will be conducted or overseen by the local investigator?
- I finished entering the information and clicked "finish." Do I need to do anything else?
- Do you have guidance materials for the IRB module of the RAP?
- How do I know if I have successfully submitted for IRB review in the RAP system?
- Why can't I create a modification in the RAP system?
- I created a modification, why is RAP not allowing me to change what I want?
- Where can I find my modifications or continuing reviews in the RAP system?
- I need to change what I selected in the Modification Scope but the RAP won't let me. What should I do?
- How do I update documents in the RAP system?
- How do I find my approval letter in the RAP system?
- I have a modification under review, but my submission is expiring soon. Can I still submit a CR/MODCR in the RAP system?
- What are some tips for communicating with RCS/IRB in the RAP?
- What is a Faculty Advisor Ancillary Review?
General Information
- Why is human subject research review important?
- I have already completed research for a class. Now I want to use this research more broadly. What do I do?
- I am not sure if my study needs to go through review. What should I do?
- I think my research qualifies as exempt. Does that mean I do not need to submit a protocol for review?
- How do I know what to submit and if I have everything?
- If I am applying for funding when should I submit to the IRB?
- What considerations do I need to make regarding informed consent?
- What are contingencies and why do they matter?
- What can I expect from the review process?
- Can a student be a Principal Investigator?
- Do I need a faculty advisor?
- Who can be a faculty advisor?
- What are the consent requirements for exempt research?
Human Subjects Education Requirement (CITI)
- Who must complete humans subjects training requirements and how can that requirement be fulfilled?
- How do I access or affiliate my already existing CITI account with UO?
- Is CITI available in other languages?
- Are there alternatives to CITI training for engaged personnel?
Working with institutions and individuals outside the University of Oregon
- I’m working with a professor at another institution and the research has already been approved by their IRB. Do I need to obtain approval from Research Compliance Services?
- I have an approved protocol and would like to collaborate with an individual not affiliated with the University of Oregon. Can I add them to my protocol?
- Do collaborators not affiliated with the University of Oregon need to complete CITI training?
- What should I do if I or someone on my team leaves the UO?
Single IRBs (sIRB)
- What is a single IRB?
- I have an existing NIH grant. Is that subject to the new sIRB policy?
- I am currently developing a protocol that involves multiple sites. How do I determine which site should serve as the sIRB of record?
- What is the SMART IRB and can I use it?
Research Administration Portal (RAP)
Why isn't CITI training showing up for an investigator?
- Check the investigator is listed under Local Study Team Members.
- Confirm the investigator has completed the correct courses. There are only a limited number of courses that will satisfy human subjects training requirements.
- Verify that the investigator name and email in CITI are an exact match to what is listed in the RAP. To integrate properly, the individual's first name in their CITI profile must match the preferred first name as listed in DuckWeb. The last name and email address as must match what is listed in the University of Oregon database (Banner). CITI training information will not populate if all three do not match. If an individual has listed a preferred name in DuckWeb, the preferred name will display in the RAP.
- Once you have updated your CITI profile to match what is listed in the RAP, it may take several days for CITI training to populate into the RAP.
Can I have a lab assistant create a submission?
Anyone can start and complete an initial submission and list someone else as the principal investigator. However, the principal investigator or an assigned PI proxy must “submit” the study to begin the review process. Once the study is submitted, anyone listed on the research team may access the protocol and create follow-on submissions (i.e., modifications and continuing review), but only the PI and PI proxies are able to submit an application to the IRB.
Can I enter more than one principal investigator?
Only one principal investigator is allowed per study. You may add additional co-investigators on the “Local Study Team Members” page. After all the study team members are entered, the Principal Investigator can assign the co-investigator(s) as PI proxy(ies).
Where should I be uploading forms and attachments such as the application, research plan, consent form, instruments, etc.?
RCS has created a table of where to upload attachments which can be found in the human subjects research guidance library.
The most common scenarios are:
- General applications such as initial review, exempt determination, continuing review or modification applications should be uploaded on the Basic Study Information page of the RAP.
- Research Plans should also be uploaded on the Basic Study Information page of the RAP.
- Consent forms should be uploaded on the Local Site Documents page of the RAP under question #1.
- Any recruitment materials should be uploaded on the Local Site Documents page of the RAP under question #2.
- Study materials such as surveys, interview questions, other measures, or other study information such as permission letters, DUAs, etc. should be uploaded on the Local Site Documents page of the RAP under question #3.
More information and specific instructions for where to upload materials is located in each application.
I am unable to locate my department, funding agency, research location, etc. from the drop-down lists. Do you have any tips to help search?
When selecting from drop-down lists, use the percentage sign (%) as a wildcard to maximize your search results. For example, “%geography” will bring up sources with “geography” anywhere in the name.
I’m conducting on-line research. How do I answer the questions of which campus research locations the research activities will be conducted or overseen by the local investigator?
When conducting remote research, use the location you will be analyzing the data or doing the work. This may be the investigator’s office or an on-campus lab. If you are not physically on campus, use your office as the location where research will be overseen.
I finished entering the information and clicked "finish." Do I need to do anything else?
Yes, you will need to click the submit link. If you don’t see a submit button, you may not have the authority to submit in the RAP.
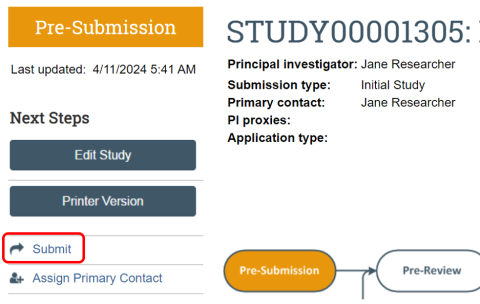
When you are satisfied with the application and are ready to submit the study for IRB review, you must choose "Submit" from the menu options on the left of the main workspace. While anyone can create a submission, only the principal investigator or a PI proxy can submit studies to the RAP module of the IRB. If you have created an initial review submission on behalf of someone else, the person listed as principal investigator must access the RAP using their DuckID and submit the study or assign a PI Proxy who then can submit the study on their behalf. The study will appear in the IRB module of RAP for the principal investigator and everyone listed as a study team member will be able to view the study, but only the PI and PI Proxies may submit an application for review. Also see the "How do I know if I have successfully submitted for IRB review?" FAQ.
Do you have guidance materials for the IRB module of the RAP?
A list of guidance documents can be found in our library. Once logged into the RAP, the guidance materials can all be found in the Help Center.
How do I know if I have successfully submitted for IRB review in the RAP system?
If you have submitted your project successfully in the RAP, it will appear in the Pre-Review state of the submissions workspace workflow map.

However, if you have NOT submitted your project, it will appear in the Pre-submission state of the submissions workspace workflow map.

In order to submit your project, please click "Submit" on the left of the screen. This will advance your submission to our office for review and change your project to the Pre-Review state in the workflow above. The Submit button location is in red in the below diagram.

Also see our RAP Guidance on how to submit your project. It also includes information about how to resubmit revisions if changes were needed.
Why can't I create a modification in the RAP system?
There may be several reasons why you are unable to create a modification to an existing study. The two most common scenarios are described below:
Scenario 1: You are not approved as the Principal Investigator (PI) or a Local Study Team Member on the project.
- Individuals who have guest access to a project in the RAP have read-only access and cannot create a modification.
- If someone is being added to the study team member list in the RAP or the PI is changing but those changes have not yet been approved, the new person won’t be able to create a modification until the change is approved.
Scenario 2: You already have modification(s) in progress.
- When you create a modification, the RAP will require you to identify the scope of the modification (“Study team member information,” “Other parts of the study,” or both). The RAP will only allow you to have one modification of each scope open at once.
- For example, you could have two modifications open at the same time if one modification has “Study team member information” selected as the scope and the other one has “Other parts of the study” selected as the scope. However, if you already have a modification created with “Study team member information” selected as the scope, you won’t be able to create another modification to change the study personnel while that one is open. If you have a modification with both scopes selected, you will not be able to create another modification of any kind until the current one is discarded or approved.
- To see the modification scope for a submission, click “View Modification/CR” on the existing modification in the RAP.
- If you’re not sure if you have a modification already in progress, see FAQ below.
For more information, also see our Modifications website, our Amending Previously Approved Research website, and the Modify Study RAP Instructions on our RAP Guidance page.
I created a modification, why is RAP not allowing me to change what I want?
Your ability to change information or materials in the RAP is based on what you select as the scope when creating a modification. Depending on the scope selected, there are three types of modifications:
- Study team member information – modifications with this selected as the scope only allow you to update the Local Study Team Members section of the RAP.
- Other parts of the study – modifications with this selected as the scope allow you to change your study documents (e.g., research plan, recruitment, consent form), the PI, and other sections of the RAP application aside from the Local Study Team Members section.
- Study team member information and Other parts of the study – When both scopes are selected, you can change all sections of the RAP, including documents, the PI and the Local Study Team Members section.
Where can I find my modifications or continuing reviews in the RAP system?
See our RAP guidance on Accessing your Studies in the RAP. In order to view a modification or continuing review for a study that has already been approved, navigate to the workspace of the parent study* and click on the “Follow-on Submissions” tab located below the submissions workspace workflow map.
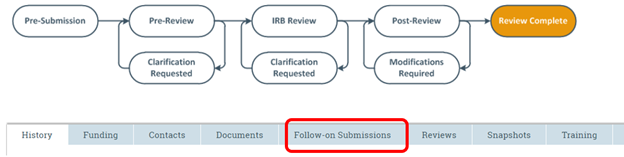
*Parent Study - When a new study is created in the RAP, that new study will become the "parent study" once approved. Unless it is a study that has migrated into the RAP from the legacy system, the parent study will have a number assigned to it that begins with "STUDY" and then a series of eight numbers (e.g., STUDY00000000). Studies that were migrated from the legacy system will be identified with an 8-digit number followed by a decimal place and three more numbers (e.g., 01012000.001). If you are on a follow-on submission such as a modification or a continuing review, you can get to the parent study by clicking on the link next to the Parent Study ID on the modification/continuing review workspace (see screenshot below).
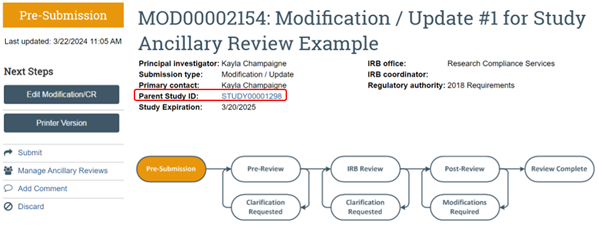
I need to change what I selected in the Modification Scope but the RAP system won't let me. What should I do?
Because the RAP will not allow anyone to change the modification scope once it is selected and the “Continue” button is clicked you will have to “Discard.” You can click “Discard” on the left side of the modification to delete the modification. Once this is complete you can create a new modification with the correct scope. If you have already submitted the modification and the submission has already been assigned to a specific IRB coordinator, please check with that coordinator (contact RCS or submit a comment) before you discard the modification.
How do I update documents in the RAP system?
When revising materials, either during the pre-review phase or when making modifications, use the “Update” button next to the document that requires revision.
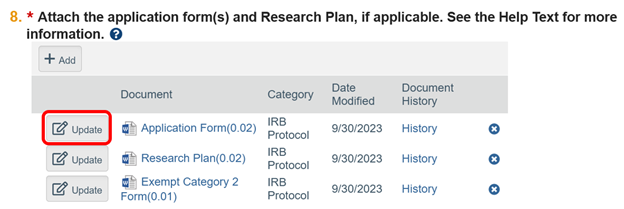
A new window will open after selecting “Update." You can select the “Choose file” button to find the updated file on your computer/Cloud/etc. The RAP smart form should automatically update the version number.
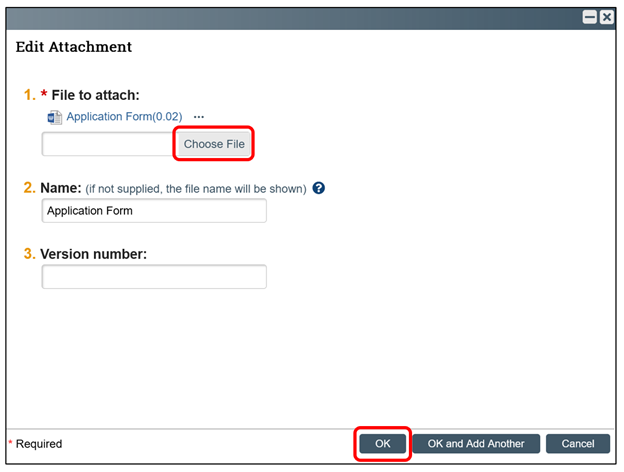
Once the updated file has been selected, click “OK” to submit the updated version.
Other Tips and Details:
- Please do NOT delete documents from the RAP and only use the “+ Add” button for new documents. The update button should be used when uploading revised documents as described above.
- The RAP system will track changes made to study documents saved in Word and correctly updated in the RAP. This allows RCS staff to review all of the changes without study teams needing to track the changes in individual documents. Unfortunately, this feature does not work on documents submitted as a PDF or image document. We encourage you to submit all documents in Word format, when possible.
- If you provide updated materials via Word documents using the update button, it is not necessary to track or highlight changes. If you are unable to provide a document in Word format, other documents and images submitted for modification should still be updated using the “Update” button, but revisions should be tracked, highlighted, or otherwise marked so that the changes can be identified and reviewed.
How do I find my approval letter in the RAP system?
You will receive an approval letter when your study is initially approved and for each subsequent submission, including modifications, continuing reviews, and reportable new information submissions. Approval letters will have some basic study information and will also include information about contingencies of the approval. It is important to review your approval letter.
There are several ways to find your approval letter:
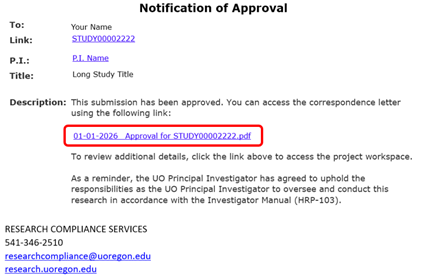
Option 1: Access your approval letter from your email notification.
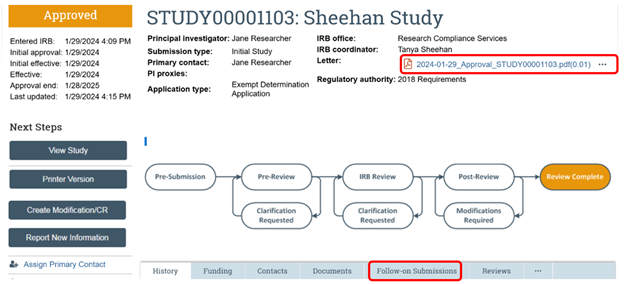
Option 2: Access your approval letter from the study workspace in the RAP.
A link to the approval letter can be found in the RAP on the upper right corner of the study workspace. Depending on which approval letter you are looking for (initial approval versus follow-on approvals for modifications, continuing reviews and reportable new information), you will need to go to different locations in the RAP. The screenshot below shows how to find the approval letter for the initial approval. If you are looking for an approval letter for a subsequent submission, click the “Follow-on Submissions” tab in the RAP, find the follow-on submission and then click on it to go to the workspace for the follow-on submission. The letter for the follow-on will be in the upper right corner of the page.
I have a modification under review, but my submission is expiring soon. Can I still submit a CR/MODCR in the RAP system?
If you have an open modification, you can still create a new continuing review (CR) submission and can likely create a modification/continuing review (MODCR) submission, as well. There are two types of modifications (MOD) in the RAP: “other parts of the study” and “study team member information.” You can only have one of each type of modification open at a time. If you already have a modification open, you will only be able to open the other type of modification as part of a MODCR. For example, if you already have a “study team member information” modification open, you will only be able to create a “other parts of the study” modification with the MODCR. However, you can always create a CR submission regardless of how many modifications you have open.
What are some tips for communicating with RCS/IRB in the RAP?
- Typically, RCS administrators will communicate to researchers through the RAP, either by requesting clarifications/revisions to your submission or asking questions using the “Add Comment” action. These actions will trigger the RAP to send automated email notifications to your UO email address. To ensure you receive these emails, please check your email settings to ensure emails from researchcompliance@uoregon.edu do not get routed to your Junk Email inbox or Other inbox.
- Please note that as of February 1st, 2024, Gmail and Yahoo have implemented stronger measures to prevent spam and improve email security, resulting in the potential loss of forwarded emails. If you had previously enabled automated forwarding from your UO email account to another account (e.g., Gmail or Yahoo), this should be disabled to ensure you are receiving the automated RAP notification emails. Instructions for disabling a UOmail forward are available in the UO Service Portal.
- When using the “Add Comment” action in the RAP, please be sure to select “IRB Coordinator” under Who should receive an e-mail notification? to ensure that RCS is notified of your comment. Otherwise, we will not know you have commented.
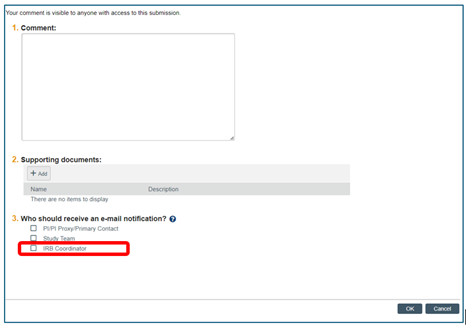
- If you added a comment in the RAP and you have not heard back, please send us an email at researchcompliance@uoregon.edu.
- Additional information can be found in the RAP Quick Reference – What to Expect guidance document.
What is a Faculty Advisor Ancillary Review?
Student researchers are only eligible to act as Principal Investigator under the oversight of a Faculty Advisor. To confirm the Faculty Advisor’s approval of the research and willingness to assume responsibility, the Faculty Advisor must complete an “Ancillary Review” in the RAP. In order to complete the Ancillary Review, the Faculty Advisor must be listed in the Study Team Members section and be assigned as a PI Proxy for the study. For more information about the Ancillary Review and how to assign this review to a Faculty Advisor, please visit the Student Led Research and Faculty Advisors page.
General Information
Why is human subject research review important?
Ethical and responsible conduct of human subject research is essential for excellence and public trust in science. The review of human subject research is ethically important to protect the rights and welfare of individual participants and ensure research integrity. The regulations that govern human subject research are based on ethical principles and guidelines that arise from issues that have occurred historically from the conduct of research with human subjects. For more information about regulations and ethical standards, see our Regulations and UO Policy webpage.
I have already completed research for a class. Now I want to use this research more broadly. What do I do?
If the initial project was intended for in-class purposes only (e.g., for a class assignment) and you now want to use information obtained for a broader purpose, the intent has shifted. If your work meets the definition of both “human subject” and “research,” you must apply for IRB review or an exempt determination. For additional information, including the definition of “human subjects research” see our Determine if your Project Requires IRB Review webpage.
I am not sure if my study needs to go through review. What should I do?
Research that meets the definition of “human subjects research” requires review. In order to determine if your research meets this definition, please complete the Human Subjects Determination Worksheet available on our Determine if your Project Requires IRB Review webpage. If you still have questions or would like formal documentation that your study is not considered human subjects research, please submit the completed form (including the last page) to Research Compliance Services via the research administration portal (RAP).
I think my research qualifies as exempt. Does that mean I do not need to submit a protocol for review?
In addition to both federal and institutional requirements, basic ethical standards apply to exempt research. Exempt research must be submitted to Research Compliance Services to verify the exempt determination and ensure these standards are met. For information on applying for an exempt determination, see our Application for Research with Human Subjects webpage.
How do I know what to submit and if I have everything?
- If you are starting a new study, our Getting Started webpage has information about what documents are required and other information or documents that may be needed, depending on the type of study you are submitting.
- You should submit a corresponding application with each submission (e.g., Initial Application, Modification Application, and Continuing Review Application). A checklist of materials that are required to be submitted are included within each application.
- You can find all of the required documents on our Applications, Forms, and Guidance webpage, including a checklist for what to submit for initial and exempt studies.
- Our RAP Attachment Guidance will help you determine where each document should be submitted.
- Please email ResearchCompliance@uoregon.edu with any questions.
If I am applying for funding when should I submit to the IRB?
Ideally, a protocol should be submitted when sufficient information about the human subjects activities covered by the grant can be included in the submission. However, some funders require documentation from the IRB that proposed human subjects activity meets federal regulatory criteria and that the investigator is aware of their obligations. In cases when the protocol activities are in development, the funder may accept documentation such documentation via an Approval-in-Principle. Research Compliance Services will work with individual investigators to ensure that appropriate review and documentation of review is available as a part of the grant application process. For additional information, see our Approval-in-Principal webpage.
What considerations do I need to make regarding informed consent?
Before involving a human subject in research, it is ethical to obtain informed consent in most cases. Unless certain criteria are met for a waiver when conducting non-exempt human subject research, informed consent is required. It is important to remember that informed consent is a process, not just a form. The process should be free of coercion or undue influence and provide information in a language understandable to the participant with sufficient opportunity for the potential participant to make an informed decision about whether to participate in the research. For more information about informed consent, including the informed consent process and documentation requirements, see our Informed Consent webpage.
What are contingencies and why do they matter?
You may see a section in your approval letter that refers to contingencies that apply to your research. Contingencies are requirements that must be met for the IRB approval to be fully satisfied or for other compliance requirements. They can include a variety of requirements, but some common examples are study team members who have expired or missing CITI certifications or submission of letters of agreement from community partners. Contingencies may limit who can conduct research or may limit all or parts of your research study from being implemented until the contingency is satisfied. It is important to read your entire approval letter to ensure you do not have any contingencies. You can always contact RCS (ResearchCompliance@uoregon.edu) if you have questions about how to satisfy your study contingency.
What can I expect from the review process?
RCS reviews materials in the order they are submitted and by submission type. Review times can also vary depending on the complexity of the study, how complete the submission materials are, and how long it takes for the study team to respond to requested revisions. There is a visual timeline on each submission page that shows where the submission is in the review process. For more information about the different steps in the review process and the timelines for review, please see RCS The Review Process: What to Expect webpage.
Can a student be a Principal Investigator?
Undergraduates, graduates, and post-doctoral scholars are all eligible to act as Principal Investigators of a research study. However, they are required to have a Faculty Advisor to provide oversight. For more information, please visit the Student Led Research and Faculty Advisors page and the Principal Investigator Eligibility, Faculty Advisors, and Research Personnel Requirements page.
Do I need a faculty advisor?
If you are a student (undergraduate or graduate) or a post doctoral scholar, you will need a faculty advisor. For more information, please visit the Student Led Research and Faculty Advisors page and the Principal Investigator Eligibility, Faculty Advisors, and Research Personnel Requirements page.
Who can be a faculty advisor?
- Faculty members with a tenure related and non-tenure track appointment are eligible to act as faculty advisors as long as they meet the qualifications of a Principal Investigator.
- Faculty members with courtesy or pro-tem appointments can only serve as faculty advisors if they meet the qualifications of Principal Investigator, will maintain their appointment for the duration of the student’s project, and have department supervisory approval. In some circumstances, department supervisory approval may be needed to ensure the research is within the scope of the courtesy appointment. If it is needed, that approval should be included in the RAP (Local Site Documents, Other attachments).
- Faculty members with emerita appointments can also act as faculty advisors for student research as long as they meet the qualifications of a Principal Investigator and the research being conducted is on behalf of their affiliation with the UO.
- For more information, please visit the Student Led Research and Faculty Advisors page and the Principal Investigator Eligibility, Faculty Advisors, and Research Personnel Requirements page.
What are the consent requirements for exempt research?
The UO requires some basic consent information to be shared with prospective research participants when the research involves any interaction or intervention. The consent process and the consent form for exempt research may be brief and may not include all of the consent elements required for expedited and full board studies. At minimum, an exempt consent process and consent form for an exempt study should include:
- A statement that the activity involves research.
- A statement that participation is voluntary.
- A brief description of what participants will be asked to do for research purposes.
- Whom to contact with questions (generally the principal investigator).
For more information, please visit the Informed Consent guidance page.
Depending on your study procedures and the sensitivity of your data, we also suggest that you consider including:
- A description of any compensation to participants. For more information, please visit the Compensation for Participation in Research guidance page.
- A brief description of whether identifiers will be collected and a how you will protect the confidentiality of the data.
Human Subjects Education Requirement (CITI)
Who must complete human subjects training requirements and how can that requirement be fulfilled?
All research personnel working with participants and/or identifiable participant data, as well as those unaffiliated with the University of Oregon and faculty members advising students, must be listed on the protocol and maintain current human subjects training. This requirement can be satisfied by completing either the Biomedical Researchers or the Social-Behavioral-Educational Researchers learners groups offered through CITI. These learner groups can be found under the Protection of Human Research Subjects training curriculum.
Please note, there are a wide variety of courses offered through CITI, but only the Biomedical Researchers or the Social-Behavioral-Educational Researchers courses will satisfy the training requirement for University of Oregon researchers. For more information, see our Human Subjects Education Requirement webpage.
How do I access or affiliate my already existing CITI account with UO?
UO faculty, students, and staff can access the CITI Program using their DuckID through the Single Sign On. It is important to note that for the research administration portal (RAP) to access CITI training, the name and email address found on the CITI website must be an exact match to that used in the RAP.
For individuals not affiliated with the University of Oregon, CITI accounts can be created and accessed through the CITI website. Once logged in, users can select "Affiliate with an Institution" to either select or add the University of Oregon to their user profile.
If you have previously completed CITI training under another institutional affiliation, any courses in common with the UO requirement will automatically count towards completion of the UO requirement. You will need to affiliate with the University of Oregon on the CITI website and update your CITI profile with your UO email. Verify that your name and email in CITI match to what is listed in the RAP (preferred first name, last name, and UO email). For the RAP to integrate properly, the individual's first name in their CITI profile must match the preferred first name as listed in DuckWeb. The last name and email address as must match what is listed in the University of Oregon database (Banner). If you are having trouble accessing your existing CITI account, please contact the CITI Program Support Center directly.
Is CITI available in other languages?
Currently, CITI content is available in English and Spanish only. Although the instructions can be shown in other languages using the drop down on the top right side of the welcome page, the modules containing the educational content are only available in English or Spanish at this time. Learners who would like to access the Spanish versions of the courses will select “Spanish Language Courses” when completing the enrollment questions.
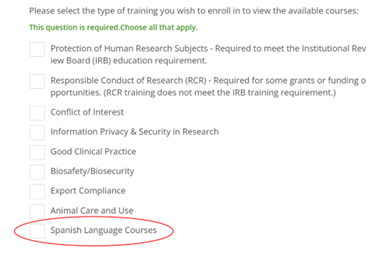
After “Spanish Language Courses” is selected, the learner can further select which courses they need to do in CITI on the following page.
CITI is not available in other languages at this time. It may be possible to use website features such as Google translate to display the content in other languages, but the translation may contain errors as automated translation software is imperfect.
If we receive a high volume of requests for certain language translations, we may be able to contact CITI to determine if a more formal translation is possible. Please reach out to researchcompliance@uoregon.edu to describe your needs.
Are there alternatives to CITI training for engaged personnel?
If the engaged personnel are UO faculty, UO staff or UO students, CITI training is a requirement. Any exceptions must be reviewed approved by RCS and/or the IRB.
If the engaged personnel are not affiliated with the UO, CITI training is still the preferred mechanism of educating them about the responsibilities and regulations associated with conducting human subjects research. CITI accounts can be created and accessed for free through the CITI website without a duck ID. Once logged in, users can select "Affiliate with an Institution" to add the University of Oregon to their user profile and complete the UO CITI training.
However, in cases where completing the required CITI training is a hardship (e.g., unable to access internet, language barriers), researchers may propose alternative training for consideration. If requesting alternative training for a non-UO affiliated individual, please provide the following details within your IRB submission (Research Plan - Investigator Qualifications, Roles, and Training section):
- A description of why they are unable to complete the required CITI training or why it would be a hardship for the individual(s).
- A description of their role in the research (e.g., obtaining consent, involved in interactions/interventions with participants, only using/analyzing identifiable private information)
- A description of the alternative training you or others plan to provide in lieu of CITI. For example, some researchers have used the free human research protection training offered by OHRP while others have created other content based off of CITI. Provide an outline of the content to be provided and any other details/slides/resources you will use.
- A description of how the alternative training is appropriate for the role that will be held by the personnel. For example, someone who will be conducting the consent process would need to have different training than someone only working with identifiable private data. Both individuals would need to be trained on the research plan (e.g., to ensure they follow the approved protocol and know they cannot deviate from what was approved), protecting participant privacy, how to store data in a confidential manner and recordkeeping requirements but the person obtaining consent would also need to have a comprehensive understanding of concepts such as the voluntary nature of the research, how to minimize undue influence, how to explain the research concepts and answer questions, how consent must be documented, how to assess comprehension, and other similar topics.
- A description of who will conduct the alternative training.
Working with institutions and individuals outside the University of Oregon.
I’m working with a professor at another institution and the research has already been approved by their IRB. Do I need to obtain approval from Research Compliance Services?
It is possible that the University of Oregon enter into an agreement with another institution to rely on their IRB review. If you would like to request that the University of Oregon rely on the IRB review of another institution, see our Collaboration in Research webpage.
I have an approved protocol and would like to collaborate with an individual not affiliated with the University of Oregon. Can I add them to my protocol?
If the collaborator is affiliated with an institution has its own IRB, we will need either (a) to enter into an agreement that allows their institution to rely on University of Oregon’s IRB review, or (b) documentation of review approval from their IRB.
If the collaborator is unaffiliated with an IRB, then they will have to sign an Individual Investigator Agreement (IIA).
In either case, you will have to submit an amendment application and update any applicable protocol materials. For more information, see our Collaboration in Research webpage.
Do collaborators not affiliated with the University of Oregon need to complete CITI training?
If the individual is affiliated with an institution that has an IRB, the University of Oregon will accept current training in accordance with their institutional policies. If the individual is unaffiliated, they will have to complete University of Oregon CITI training requirements. Collaborators can login to the CITI website, affiliate with the University of Oregon, and complete the training required by University of Oregon researchers. Investigators do not need a university ID or email to affiliate with the University of Oregon on the CITI website. For more information, see our Human Subjects Education Requirement webpage.
Alternatives to CITI training can be considered with justification and a complete alternative training plan. Please also see the Human Subjects Education Requirement (CITI) section above for more information.
What should I do if I or someone on my team leaves the UO?
Consider whether the study is complete or if it needs to be continued.
- If the study is complete, (i.e., permanently closed to new enrollment, all interactions/interventions are complete, data collection is complete, analysis of private identifiable information is complete and all existing data/samples are either destroyed or all identifiable information has been destroyed so it is not possible for you or anyone else to link identities to the data/samples), you will need to submit a continuing review (CR) to close your study. See our Continuing Review/Study Closure (RAP Instructions) for more information. The study closure application that you will include in the submission can be found on our Forms and Guidance page.
- If the study is continuing, the answer depends on who has left the institution.
- If you are the Principal Investigator (PI) in charge of the study and you have left the UO for another institution that has an IRB, please reach out to the IRB at your new institution to determine what they will require in order to transfer your project over to their oversight. Contact RCS after you have talked with them to discuss your options (researchcompliance@uoregon.edu).
- If you are the Principal Investigator (PI) in charge of the study and you have left the UO but your new institution does not have an IRB, it may not be possible for you to remain PI. If it is possible to select a new PI, you may be able to remain on the study as an independent external investigator. To be an independent external investigator, you will need an Individual Investigator Agreement (IIA), an external personnel form, and a modification will be needed to the project to update the PI in the RAP and the various study materials (e.g., research plan, recruitment, consent forms etc.) and to get your IIA, external personnel form, and CITI completion report added to the study record. For more information, see our collaborations page and our modification page.
- If you are the PI and one of your existing study team members has left the UO and they are no longer working on the project, submit a modification to remove the personnel who left. If they were listed on any of your study materials (e.g., research plan, recruitment materials, consent forms), you will need to select both “study team member information” and “other parts of the study” within the Modification Scope so you can update your study materials in addition to the information in the Local Study Team Members section. For more information, also see our Modifications website, our Amending Previously Approved Research website, and the Modify Study RAP Instructions on our RAP Guidance page.
- If you are the PI and one of your existing study team members has left the UO and they want to continue working on the project, what you do will depend on a number of factors:
- Engagement: Is this study team member still going to be engaged in the human subjects research (e.g., obtaining consent, interacting/intervening with participants, using/analyzing identifiable private information)? If not, they can just be removed from the study. If they continue to be engaged, continue to other options below.
- Affiliation: Is the engaged external team member going to be affiliated with an institution that has its own IRB? If not, continue to the last option below. If they are going to be affiliated with an institution that has its own IRB, the UO IRB may be able to enter into a reliance agreement with their new institution such that their IRB can rely on the UO IRB for review and approval. Ask your team member to check in with the IRB at their new institution to see if that would be possible. If not, they may need to obtain approval from the IRB at their new institution. Contact RCS after you have talked with them to discuss your options (researchcompliance@uoregon.edu).
- Independent Investigator: If the external study team member is not affiliated with an institution that has its own IRB, it may be possible for them to be added as an external investigator. To be an independent external investigator, you will need an Individual Investigator Agreement (IIA), an external personnel form, and a modification will be needed to the project to update the PI in the RAP and the various study materials (e.g., research plan, recruitment, consent forms etc.) and to get your IIA, external personnel form, and CITI completion report added to the study record. For more information, see our collaborations page and our modification page.
- If you are a student and your faculty advisor has left the UO, you will need to find a new faculty advisor. Once you have identified your new faculty advisor (FA), submit a modification to change the faculty advisor in the RAP. When creating the modification, consider whether your FA is listed on any of your study materials (e.g., research plan, recruitment materials, consent forms). If so, you will need to select both “study team member information” and “other parts of the study” within the Modification Scope so you can update your study materials in addition to the information in the Local Study Team Members section. For more information, also see our Modifications website, our Amending Previously Approved Research website, and the Modify Study RAP Instructions on our RAP Guidance page.
Single IRBs (sIRB)
What is a single IRB?
A single IRB (sIRB) is the IRB of record that oversees the ethical review for all sites participating in a multisite study.
When is the use of a sIRB required?
Effective January 25, 2018, studies wholly or partially funded by the NIH require the use of an sIRB for the review of all non-exempt, multi-site, domestic, human subjects research. Additionally, effective January 20, 2020, under the revised Common Rule, most federally supported or conducted cooperative studies that meet the criteria for non-exempt human subjects research and involve more than one site require sIRB review, unless:
- Cooperative research for which more than a single IRB review is required by law (including tribal law passed by the official governing body of an American Indian or Alaska Native tribe); or
- Research for which any Federal department or agency supporting or conducting the research determines and documents that the use of a single IRB is not appropriate for the particular context.
I have an existing NIH grant. Is that subject to the new sIRB policy?
The NIH sIRB requirement applies to all competing grant applications, including new, renewal, revision or resubmission, submitted on or after January 25, 2018.
I am currently developing a protocol that involves multiple sites. How do I determine which site should serve as the sIRB of record?
This depends on a multiple number of factors including the experience and expertise of the IRB reviewing the research and funder requirements. Research Compliance Services can help determine which IRB will be the best fit for your research.
What is the SMART IRB and can I use it?
The SMART IRB is a service designed to streamline IRB review for multisite studies. The platform allows investigators and institutions to request, track, and document reliance agreements on a study-by-study basis. Although it is not a requirement, the University of Oregon will use the SMART IRB agreement when possible. To learn more about the SMART IRB reliance agreement and confirm whether your collaborators have signed on, please visit Smart IRB.
Please note that using the SMART IRB online reliance system or the SMART IRB Letter of Acknowledgement (LOA) replaces the need for an IRB Authorization Agreement (IAA). Not all institutions use SMART IRB and external collaborators may have preferences regarding how they initiate reliance agreements. UO is flexible and will work with external collaborators regarding their preferences. Before using SMART IRB, we recommend that you speak with your external collaborators regarding the other IRB’s preference and/or contact RCS so we can help facilitate those interactions. Researchers can also review our Collaborations in Research page and contact RCS for more information if they have questions about SMART IRB.
