The Office of the Vice President for Research and Innovation (OVPRI) has embarked on a transformative project to improve research administration services and efficiency for our faculty, staff, and students who interact with our office. User guidance, portal access, and project updates are provided below.
RAP Access
Reminders for submitting and resubmitting IACUC and IRB applications:
- If you are working on a new submission, make sure you click "Submit" on the left-hand side of the page in the RAP (under Next Steps) to submit your study for review. The review process cannot begin until this step is complete.
- If you were asked to make changes during IRB or IACUC review, make sure you click "Submit Response" on the left-hand side of the page in the RAP (under Next Steps) to resubmit your study for review. The review process cannot continue until this step is complete.
RAP Transition Updates
Launched in January of 2020, the Research Administration Portal (RAP) is dedicated to helping you manage all your research administration activities, including:
- AGREEMENTS: Research-Related Agreement Management
(Huron Research Suite version 12) - GRANTS: Pre-award and Post-award Management - coming 2026-27
(Huron Research Suite version 12)
- COI: Conflicts of Interest and Commitment
(Huron Research Suite version 10.5) - IACUC: Animal Research
(Huron Research Suite version 10.5) - IRB: Human Subjects Institutional Review Board
(Huron Research Suite version 10.5)
- ECC: Effort Certification Compliance
(Huron Research version ECC)
Each of these modules is developed and implemented in partnership with the campus research community. On this page, you will find an overview of the project as well as updates as the project moves forward. Please reach out to rapinfo@uoregon.edu if you have any questions or feedback.
September 2025
RAP Agreements
RAP Agreements has been live since April 2025. Sponsored Projects Services (SPS) is using the system for Sponsored Research Agreements. Industry, Innovation, and Translation (IIT) is using it for Confidential/Non-Disclosure Agreements, Data Use Agreements, and Material Transfer Agreements. New Training resources and workflow guidance will be shared with the campus community this fall to support broader use. For questions, contact agreements@uoregon.edu.
RAP Grants in Iteration Phase
RAP Grants (Huron Research Suite version 12) has entered the iteration phase. Integration work with Banner and data conversion from E-PCS and Banner will begin in 2026. Go-live is currently targeted for late 2026, with details on timing and phased roll-out still being refined. As implementation moves forward, engaging the campus community will be key. Demos, feedback sessions, and regular updates will expand in 2026 to ensure faculty and staff can help shape the system. The project team will continue refining workflows, preparing for data conversion, and planning for the transition from E-PCS, with training and change communications to follow. For questions, contact rapinfo@uoregon.edu.
April 2025
RAP Agreements: Go-Live April
The new RAP Agreements module is live. Sponsored Projects Services is using the new production site to manage Sponsored Research Agreements and will begin onboarding DGAs through a phased approach throughout April. Industry, Innovation, and Translation will be using the RAP Agreements module for Confidential/Non-Disclosure Agreements, Data Use Agreements, and Material Transfer Agreements later in April. For questions, please email agreements@uoregon.edu.
RAP Grants in Onboarding Phase
The implementation of the RAP Grants module is underway. The RAP Grants solution will streamline pre-award processes, repurpose data for new submissions, automate manual tasks, and introduce critical post-award non-financial capabilities (ePCS will eventually be sunsetted). RAP Grants and RAP Agreements will operate on a new major version of the software, Version 12. The other RAP modules (COI, IACUC, IRB) will be upgraded to version 12 next year, and eventually ECC, to create a single, integrated platform. The implementation timeline for RAP Grants is estimated at approximately 14 months and there will be a phased launch approach and a clear transition plan between ePCS and RAP Grants. Project updates will posted here. If you have any additional questions, please email rapinfo@uoregon.edu
February 2025
The Next Rap Project: RAP Grants
In early March, the Office of the Vice President for Research and Innovation (OVPRI) will kick off the implementation of the Grants module as part of the Research Administration Portal (RAP). The RAP Grants solution will streamline pre-award processes, repurpose data for new submissions, automate manual tasks, and introduce critical post-award non-financial capabilities. RAP Grants and RAP Agreements will operate on a new major version of the software, Version 12. The other RAP modules (COI, IACUC, IRB) will be upgraded to version 12 next year to create a single, integrated platform. The implementation timeline for RAP Grants is estimated at approximately 14 months. Project updates will posted here. If you have any additional questions, please email rapinfo@uoregon.edu.
RAP Agreements: Go-Live April
The new RAP Agreements module is launching soon, providing a unified platform for managing all research-related agreements. In the coming weeks, outreach for testing sessions, demos, and training sessions will occur. Go-live is scheduled for early April. For questions or to participate in testing, demos, or training, please email rapinfo@uoregon.edu.
November 2024
The Next Rap Project: RAP Agreements
In October, the Office of the Vice President for Research and Innovation (OVPRI) kicked off the implementation of a new research administration tool designed to serve as a centralized repository for research contracts and agreements. As part of the broader suite of research admin solutions at the University, the Research Administration Portal (RAP) Agreements solution will streamline processes across the institution, providing a unified platform for managing all research-related agreements.
RAP Agreements is scheduled to go-live at the end of March in early April 2025. Initially, RAP Agreements will operate as a stand-alone solution, as it built using a new major version of the software, Version 12. The system will be integrated with the broader RAP suite once the other modules have been upgraded to version 12 (TBA).
The RAP Agreements project team is currently in the onboarding phase, meeting regularly with the vendor to review product functionality, identify system configurations and business process changes that will be required for system implementation. Project updates will posted here. If you have any additional questions, please email rapinfo@uoregon.edu.
RAP ECC (Effort Certification Compliance) Live
RAP ECC has been live since August and had a successful first round of statement certifications for Q2 of 2024. Kudos to the project team, DGAs, and PIs for all their hard work and attention to detail with the transition from EPCS to RAP ECC. For more information and resources, visit the payroll certifications webpage.
August 2024
RAP ECC (Effort Certification Compliance): Go-Live Aug 6
UO's new effort certification tool, RAP ECC, is live as of August 6, 2024. RAP ECC supports the processes and policies around effort reporting, certifying to granting agencies that the personnel paid on sponsored awards reflects their effort performed on the project. Live training and Q&A sessions for campus research administrators and researchers will continue throughout August. Q2, 2024 and going forward, EPCS will no longer be used for effort certification. For more information and resources, visit the payroll certifications webpage.
RAP COI, IACUC, IRB downtime Aug 21-23; Upgrade Go-Live Aug 23
The COI, IACUC, and IRB modules of the RAP will be upgrading to version 10.5. The teams have been busy with testing and preparations. Sites will be offline beginning at 2 p.m. PST on Wednesday, August 21, 2024. Sites are expected to be back online with version 10.5 by approximately 5 p.m. on Friday, August 23, 2024. The upgrade includes many bug fixes, performance enhancements, and improvements. If you have any questions, please email rapinfo@uoregon.edu.
June 2024
RAP ECC (Effort Certification Compliance)
UO's new effort certification tool, RAP ECC, is launching soon. RAP ECC will support the processes and policies around effort reporting, certifying to granting agencies that the personnel paid on sponsored awards reflects their effort performed on the project. The testing phase has been completed, and the pilot phase is now in progress. Demos and training sessions for campus research administrators and researchers will take place in July. The system will officially go live on August 6, with pre-review opening for the second quarter of 2024.
If you have any additional questions, please email rapinfo@uoregon.edu.
RAP Upgrade 10.5: COI, IACUC, IRB
The COI, IACUC, and IRB modules of the RAP will be upgrading to version 10.5. Kick-off took place on May 28 and the teams are currently performing system testing through June. Go-live for version 10.5 is scheduled for end of business day on August 23, 2024, with sites offline August 21-23. The upgrade includes many bug fixes, performance enhancements, and improvements. See October update below for highlights. If you have any questions, please email rapinfo@uoregon.edu.
April 2024
RAP ECC (Effort Certification Compliance)
As part of the Research Administration Portal, implementation of RAP ECC is underway. This new effort certification tool that will support the processes and policies around effort reporting, the method of certifying to granting agencies that the personnel paid on sponsored awards reflects their effort performed on the project. RAP ECC is currently in the pilot phase with a group of Departmental Grant Administrators (DGAs) and Principal Investigators (PIs). Demos followed by training will be happening with the campus community beginning in June. RAP ECC is scheduled to go live in August 2024.
If you have any additional questions, please email rapinfo@uoregon.edu.
RAP Upgrade 10.5: COI, IACUC, IRB
The COI, IACUC, and IRB modules of the RAP will be upgrading to version 10.5. The project team and the vendor have begun upgrade work and the kick-off is scheduled for early May 2024. Go-live for version 10.5 is scheduled for early August 2024. The upgrade includes many bug fixes, performance enhancements, and improvements. See October update below for highlights.
If you have any questions, please email rapinfo@uoregon.edu.
February 2024
RAP ECC (Effort Certification Compliance)
Implementation of the RAP ECC, the new effort certification tool that will support the processes and policies around effort reporting (the method of certifying to granting agencies that the personnel paid on sponsored awards reflects their effort performed on the project) is ahead of schedule and making great strides. This new tool, RAP ECC, is scheduled to go-live in August 2024.
The project team has been working closely with the vendor to review the RAP ECC system and identify system configurations and/or business process changes that may be required for system implementation. In March and April, outreach will begin with Departmental Grant Administrators (DGAs) and departments, and will continue throughout the next few months. If you have any additional questions, please email rapinfo@uoregon.edu.
RAP Upgrade 10.5: COI, IACUC, IRB
The COI, IACUC, and IRB modules of the RAP will be upgrading to version 10.5. Due to vendor product release issues, the upgrade project has been rescheduled to start in April 2024 and is expected to conclude in July 2024. The upgrade includes many bug fixes, performance enhancements, and improvements. See below for highlights.
If you have any questions, please email rapinfo@uoregon.edu.
October 2023
The Next RAP Project: RAP ECC
In October, the Office of the Vice President for Research and Innovation (OVPRI) is kicking off the implementation for a new effort certification tool that will support the processes and policies around effort reporting, the method of certifying to granting agencies that the personnel paid on sponsored awards reflects their effort performed on the project. This new tool, RAP ECC, is scheduled to go-live in summer 2024.
The project team will be meeting with the vendor to review the RAP ECC system and identify system configurations and/or business process changes that may be required for system implementation.
The project team will provide monthly updates about the progress of RAP ECC implementation here. If you have any additional questions, please email rapinfo@uoregon.edu.
RAP Upgrade 10.5: COI, IACUC, IRB
The COI, IACUC, and IRB modules of the RAP will be upgrading to version 10.5. This upgrade project will begin in January 2024 and is anticipated to be completed in March 2024. The upgrade includes many bug fixes, performance enhancements, and improvements. Highlights include:
COI
- Improvements to disclosure profile interface
- Improved Central Actions allow COI staff to update write-in entities
- New functionality to allow COI staff to assign categories to users
IACUC
- New setting to authorize IACUC staff to edit, delete, and resolve threads for reviewer notes authored by other users
- Related procedures are automatically added to an experiment on a submission
- New questions added in Experiments, Protocols
- New "Pre-Submission Assistance" feature which enables the PI or protocol team to request assistance from an IACUC coordinator veterinarian in Pre-Submission
- IACUC AAALAC report was reformatted to display more detailed information.
- Added three new questions to IACUC Settings/Advanced Settings/Other to select the procedure types to consider when validating if a protocol has special considerations. These special considerations are displayed in the AAALAC report.
IRB
- A new setting that allows the IRB Data Manager to add, edit, and disable the RNI categories
- Related PI or PI Proxies can now Edit and Submit
- Expanded ability to edit RNIs in the Action Required State
- Enabling IRB staff to administratively close submissions
- Enhanced AAHRPP report template to more closely align with AAHRPP's updated report format
- A new setting to enable PIs to submit study updates on external single site studies
If you have any questions, please email rapinfo@uoregon.edu.
April 2023
RAP COI Project
RAP COI went live on April 21, 2023.
If you have any questions, please email rapinfo@uoregon.edu.
March 2023
RAP COI Project
The COI project team continues to focus their efforts on go-live readiness and training. The go live date is scheduled for April 20, 2023.
If you have any questions, please email rapinfo@uoregon.edu.
February 2023
RAP COI Project
The iteration phase of the project (to test system requirements) is complete, as well as User Acceptance Testing. The COI project team continues to focus their efforts on go live readiness. Training preparation is in progress. The tentative go live date is April 20, 2023.
If you have any questions, please email rapinfo@uoregon.edu.
January 2023
RAP COI Project
The iteration phase of the project, to test the system requirements identified during Onboarding, is nearly complete. User Acceptance Testing is tentatively scheduled for the week of February 6, 2023.
If you have any questions, please email rapinfo@uoregon.edu.
December 2022
RAP COI Project
The tentative go live date is February 2023; however, the go live date may be pushed out to March. The iteration phase of the project to develop and test the system requirements continues. User Acceptance Testing is tentatively scheduled for the week of January 17, 2023.
If you have any questions, please email rapinfo@uoregon.edu.
November 2022
RAP COI Project
The tentative go live date is February 2023. The iteration phase of the project to develop and test the system requirements continues. User Acceptance Testing is tentatively scheduled for the week of January 17, 2023.
If you have any questions, please email rapinfo@uoregon.edu.
October 2022
RAP IACUC Project
The RAP IACUC module is now live.
Office hours and formal training sessions are available for researcher training.
RAP COI Project
The initial project schedule is complete with a projected go live of February 2023.
The iteration phase of the project to develop and test the system requirements continues.
If you have any questions about either of the projects, please email rapinfo@uoregon.edu.
September 2022
RAP IACUC Project
The final automated data conversions into the IACUC production system were completed on September 14. The Project Team is updating the converted protocols with data that was not loaded during the final automated data conversion.
Access to the IACUC module by the researchers will be available once the protocols are updated.
RAP COI Project
Final adjustments to the project schedule are in progress. The iteration phase of the project to develop and test the system requirements continues
If you have any questions about either of the projects, please email rapinfo@uoregon.edu.
August 2022
RAP IACUC Project
Due to issues encountered with the data conversions, the system launch has been delayed until early to mid September.
In the mean time, the Project Team continues to focus on launch readiness tasks such as loading the application help text and preparing for training.
RAP COI Project
The Project Team is evaluating Huron tools that may be used to support the metrics reporting requirements.
The iteration phase of the project to develop and test the system requirements continues. The Project Team will test the requirements as they are developed.
If you have any questions about either of the projects, please email rapinfo@uoregon.edu.
July 2022
RAP IACUC Project
The IACUC the project team continues to focus their efforts on go live readiness.
Training preparation is in progress and training will be scheduled for the middle of August.
The IACUC system launch has been scheduled for August 22.
RAP COI Project
With the exception of COI metrics reporting, the system requirements identified during the onboarding phase have been finalized and approved.
The iteration phase of the project to develop and test the system requirements is in progress. The project team will test the requirements as they are developed with the objective of having the development and testing completed by the end of August or early September.
If you have any questions about either of the projects, please email rapinfo@uoregon.edu.
June 2022
RAP IACUC Project
The RAP IACUC the project team continues to focus their efforts on go live readiness (final setups) and training preparation for the system launch currently scheduled for later this summer.
RAP COI Project
The system requirements identified during the onboarding phase will be finalized and approved by the end of June.
The development phase of the project to develop and test the system requirements will begin in July.
If you have any questions about either of the projects, please email rapinfo@uoregon.edu.
May 2022
RAP IACUC Project
The RAP IACUC project team continues to focus on the final setups and training preparations for the IACUC module implementation this summer.
RAP COI Project
The RAP COI project team continues to refine the proposed business process changes.
The system requirements identified during the onboarding phase are being clarified in preparation for the development phase in July.
If you have any questions about either project, please email rapinfo@uoregon.edu.
April 2022
COI Project
The COI project team completed a four-week onboarding process with the vendor to identify system requirements and proposed business process changes. Over the next few months, the project team will review the system requirements and proposed business process changes with stakeholders in the research community for feedback.
If you have any questions about the COI project, please email rapinfo@uoregon.edu.
March 2022
IACUC Project
The IACUC the project team continues to focus their efforts on training preparation and go-live readiness for the system launch this summer.
The Next RAP Project: RAP COI
Last week, the Office of the Vice President for Research and Innovation (OVPRI) kicked off the implementation for a new conflict of interests (COI) tool that will support the Financial Conflict of Interest in Research policy and the Conflict of Interest, Conflict of Commitment, and Outside Activities policy. This new tool is scheduled to go-live in late 2022.
Over the next couple of weeks, the project team will be meeting with the vendor to review the COI system and identify system configurations and/or business process changes that may be required for system implementation.
The project team will provide monthly updates about the progress of the COI tool implementation. If you have any additional questions, please email rapinfo@uoregon.edu.
February 2022
User testing completed on January 28. The project team reviewed the test results and feedback from users which resulted in some minor changes to the system.
Over the next few months, the project team will focus their efforts on training preparation and go-live readiness for the system launch this summer.
January 2022
Over the past few weeks, the project team has been actively planning and preparing for user testing. User testing is set to begin today, January 18, and run through January 28.
User testing is being completed by a handful of animal researchers and IACUC staff.
The goal of user testing is to verify that the system operates as expected before moving the system into the live environment. Its purpose is to validate the end-to-end business flow by walking through real-world scenarios, identify any issues that need to be corrected prior to go-live, and help inform training.
Once user testing is complete, the project team will shift its focus to preparing for training and go-live this summer.
December 2021
After careful consideration by OVPRI leadership and the project team, the IACUC project timeline has been extended and we plan to go-live with the new IACUC tool in late summer 2022. An exact date will be communicated next year.
In adjusting the timing, we were motivated to:
- ensure investigators a smooth roll-out and transition from the current system (eAPM),
- allow the Animal Welfare Services team more time to develop and build adequate support resources for the new tool, and
- provide the team time to prepare for the AAALAC International accreditation renewal process which will take place in the first half of 2022.
What does this mean for investigators?
Protocol submissions, renewals, and amendments will continue to take place in eAPM through the first half of 2022. This will allow the project team to migrate more information and consolidate a library of standard procedures.
Investigators and their teams will need to fully populate and validate protocols in the new system after go-live and prior to any protocol amendments or modifications.
What’s Next?
The project team will continue working on the project and will begin user testing in mid- to late-January 2022. More precise timing and information about the transition will be available in the coming months.
October 2021
The project team has developed a one-page resource summarizing the changes that will come with the IACUC module and what you can expect.

October 2021
The project team continues to work with the vendor on system configuration and development. This work will continue over the next month.
Once system configuration and development are completed, the team will begin user acceptance testing (UAT). User acceptance testing is a type of testing that is performed by end users to verify that the system operates as expected before moving the system into the production environment. UAT is an important step to perform before rolling out new systems. Its purpose is to validate the end-to-end business flow by walking through real-world scenarios, identify any issues that need to be corrected prior to go-live, and help inform training.
UAT is scheduled to begin in late-November/early-December. If you are interested in participating in user testing, email your interest to rapinfo@uoregon.edu.
Lastly, to help keep the community informed about this project and the changes the new system will introduce, the project team has updated the Animal Research page on the research website with two new pages:
September 2021
The final system requirements have been approved. The project team has started working with the vendor on an iterative system development and configuration process. This is expected to continue over the next few months.
A project update communication was sent to the IACUC community on 8/27 from Jim Slattery, Associate Vice President for Research Operations. This communication included a link to a webpage providing users with a sneak peak of the new IACUC module.
If you would like to receive monthly updates about this effort, please subscribe to the following email list: rap-iacuc-module@lists.uoregon.edu, or click to subscribe.
August 2021
The project team is still in the process of finalizing system requirements.
For the past couple of weeks, the team has been working with the vendor on a series of design sessions to finalize requirements. The final system requirements will be reviewed and approved by the Vice President for Research and Innovation based upon the recommendation of the project champions and team. After that, the team will begin working with the vendor on an iterative system development and configuration process. This process will begin shortly and will take a couple months to complete.
July 2021
The IACUC project team completed a four-week onboarding process with the vendor to identify system requirements and business process changes required for implementation.
The project team is now compiling the proposed system requirements and business process changes and will review them with stakeholders in the research community for feedback. The final system requirements will be approved by the VPRI.
Once the system requirements are approved, the vendor will begin an iterative system configuration and implementation process. This is likely to begin in late-July or early-August. Each iteration will be tested by the project team.
If you would like to receive monthly updates about this effort, please subscribe to the following email list: rap-iacuc-module@lists.uoregon.edu, or click to subscribe.
June 2021
Since the full launch of the RAP IRB module, the project is now officially closed. The project team gathered lessons learned and Research Compliance Services continues to offer trainings and provide support for the system. If you have questions about how to submit a study, contact Research Compliance Services by phone (541-346-2510) or email (researchcompliance@uoregon.edu).
A new Research Administration Portal project to implement an institutional animal care and use committee (IACUC) module kicked off last week and is underway. Over the next couple of weeks, the project team will be meeting with the vendor to review the IACUC system and identify system configurations and/or business process changes that may be required for system implementation.
As the IACUC implementation progresses, we will continue to share information with IACUC community. If you would like to receive monthly updates about this effort, please subscribe to the following email list: rap-iacuc-module@lists.uoregon.edu, or click to subscribe. Monthly updates will be posted on this webpage as well.
If you have any questions or concerns about the IACUC project, you are invited to share those by emailing rapinfo@uoregon.edu.
May 12, 2021
The Office of the Vice President for Research and Innovation (OVPRI) and Research Compliance Services (RCS) are pleased to announce that the RAP IRB module full launch is now live.
You can now submit all your IRB study transactions via the RAP IRB module.
If your active and approved legacy study was migrated to the new system, please remember that the first time a continuing review or modification is submitted to our office, some information will need to be entered and all approved study documents will need to be uploaded to complete the study record in the RAP IRB module. See our Migrated Legacy Study guidance for more details.
If you have questions about the RAP IRB module or how to submit a transaction, contact Research Compliance Services by phone (541-346-2510) or email researchcompliance@uoregon.edu.
May 10, 2021
RAP IRB Module
The full launch of the RAP IRB module is set to go-live in two days on Wednesday, May 12. Please note that on the evening of Tuesday, May 11, the RAP IRB module will be taken offline and be unavailable starting at 5pm.
As the project team gets ready for the full launch on Wednesday, below are a few points to remember:
- Beginning on Wednesday, May 12, all human subject research applications and transactions with the Research Compliance Services office will be managed through the RAP IRB module.
- All active and approved studies that are not yet in the RAP IRB module (i.e., “legacy studies”) will be migrated into the new system prior to Wednesday. No action is required to establish the study record in the system.
- The first time a continuing review or modification is submitted for a legacy study, some information will need to be entered and all approved study documents uploaded to complete the migration to the RAP IRB module.
- Training workshops are still being offered. See the training catalog for more information on dates, times, and registration links for the latest sessions.
For questions, visit the Human Subjects Research webpage or contact Research Compliance Services by phone (541-346-2510) or email (researchcompliance@uoregon.edu).
The Next RAP Project: RAP IACUC
Starting next month, the Office of the Vice President for Research and Innovation (OVPRI) will begin implementing a new institutional animal care and use committee (IACUC) tool to support animal research that will go-live in early 2022.
The new IACUC tool will allow the UO to adopt more efficient business processes and workflows that will help improve investigators research capacity and provide a more transparent and seamless workflow. Some features investigators can expect with the new tool are:
- Improved visibility as to where a protocol is in the review process. By simply logging into the system, investigators will easily see if their protocol is in the pre-review state, being reviewed by the IACUC, or in post-review.
- Integration with the American Association for Laboratory Animal Science (AALAS) for easy management of training records and requirements.
- Infrastructure for a library of standard substances and procedures that supports IACUC review, versioning, and promoting research lab procedures to standard procedures.
- A comprehensive view of research lab teams that includes team members, trainings, substances, procedures, and protocols.
If you would like to receive monthly updates about this effort, please subscribe to the following email list: rap-iacuc-module@lists.uoregon.edu, or click to subscribe.
April 2021
The full launch of the IRB module is set to go-live next month in May. As a reminder, the full launch is the second of two phases for the RAP IRB module.
At the time of the full launch in May, all human subject research applications and transactions with the Research Compliance Services office will be managed through the RAP IRB module. All active and approved studies that are not yet in RAP IRB module, which are referred to as “legacy studies”, will be migrated into the new system.
Transitioning Legacy Studies
Prior to the full launch, active and approved legacy studies will be moved into RAP IRB module. No action will be required from the Principal Investigator to establish the legacy study in the system.
Please note, however, the first time a continuing review or modification is submitted for a legacy study, some information and documents will need to be entered and/or uploaded to complete the study record in the RAP IRB module.
It is strongly encouraged that investigators with legacy studies attend a RAP training workshop.
Training Workshops
Beginning this month, Research Compliance Services (RCS) will offer several virtual, hands-on training workshops for investigators, research staff, and other stakeholders to prepare for the full launch.
- RCS will offer a workshop dedicated to supporting legacy studies during the transition of systems. In this workshop, investigators and research staff will be able to log into the system, see their migrated study in the RAP IRB module, walk through the process of submitting a modification or continuing review, and receive support for upcoming submissions.
- RCS has also developed a set of routine workshop offerings to support users with basic system orientation, preparing and submitting applications, managing studies, and more.
See the training catalog for more information on dates, times, and registration links for the latest sessions.
March 2021
The project team has completed data migration testing of all active and approved studies in preparation for the full launch. The team has now started functional testing that will run through April.
Research Compliance Services (RCS) has started working on guidance materials for investigators with migrated studies with information about what to expect with this transition. RCS has also started planning full launch training which is scheduled to begin in April.
More information about guidance materials and training dates will be sent to the IRB community via email in the coming weeks. The project team will also update this webpage with information.
February 2021
Since the soft launch of the IRB module on January 25, Research Compliance Services (RCS) has been monitoring the module to ensure the system is functioning as expected and supporting investigators with their new study submissions. If you have any questions about the IRB module, how to submit your new study, or which process to use for your transaction, contact RCS by email (researchcompliance@uoregon.edu).
The project team is now planning and preparing for the full launch of the IRB module scheduled to go-live in May.
The full launch consists of migrating all active and approved studies from the legacy IRB database to the IRB module. At that time, all investigators will submit and manage all transactions (e.g., continuing reviews, modifications, etc.) through the IRB module.
For more information and updates about this project, subscribe to the IRB Research Administration Portal email list by visiting the signup page.
January 25, 2021
The RAP IRB module is now live.
Investigators can submit their new IRB study submissions by clicking on the "IRB" button at the top of this page or by navigating to the RAP IRB module.
For more information about the phased launches, training, and who to contact for help, see the update from January 19, 2021 below.
January 19, 2021
The project team is very excited to announce the soft launch of the IRB module for new study submissions is set for Monday, January 25.
Beginning on 1/25, investigators will be able to access the IRB module to submit new IRB study submissions by clicking on the “IRB” button at the top of this page.
As a reminder, this go-live is the first of a two-phased launch of the IRB module. Investigators with new protocols should submit and manage their protocol using the new IRB module of the RAP system. Existing studies, including amendments and continuations, should continue to be managed using the current process.
The second phase will launch later this year in Spring. In preparation for the second phase go-live, Research Compliance Services (RCS) will migrate all existing studies into the system and all investigators will submit and manage all transactions through the IRB module beginning in the Spring. Precise dates, more training, and guidance to support you during the transition will be shared as we approach Spring.
Training for the IRB module is still being offered. See our training catalog for more information on dates, times, and registration links.
If you have any questions about the IRB module, contact RCS by email (researchcompliance@uoregon.edu).
December 2020
The project team has developed a one-page resource summarizing the changes that will come with the IRB module and what you can expect.
Click on the image below to view a full-screen version.
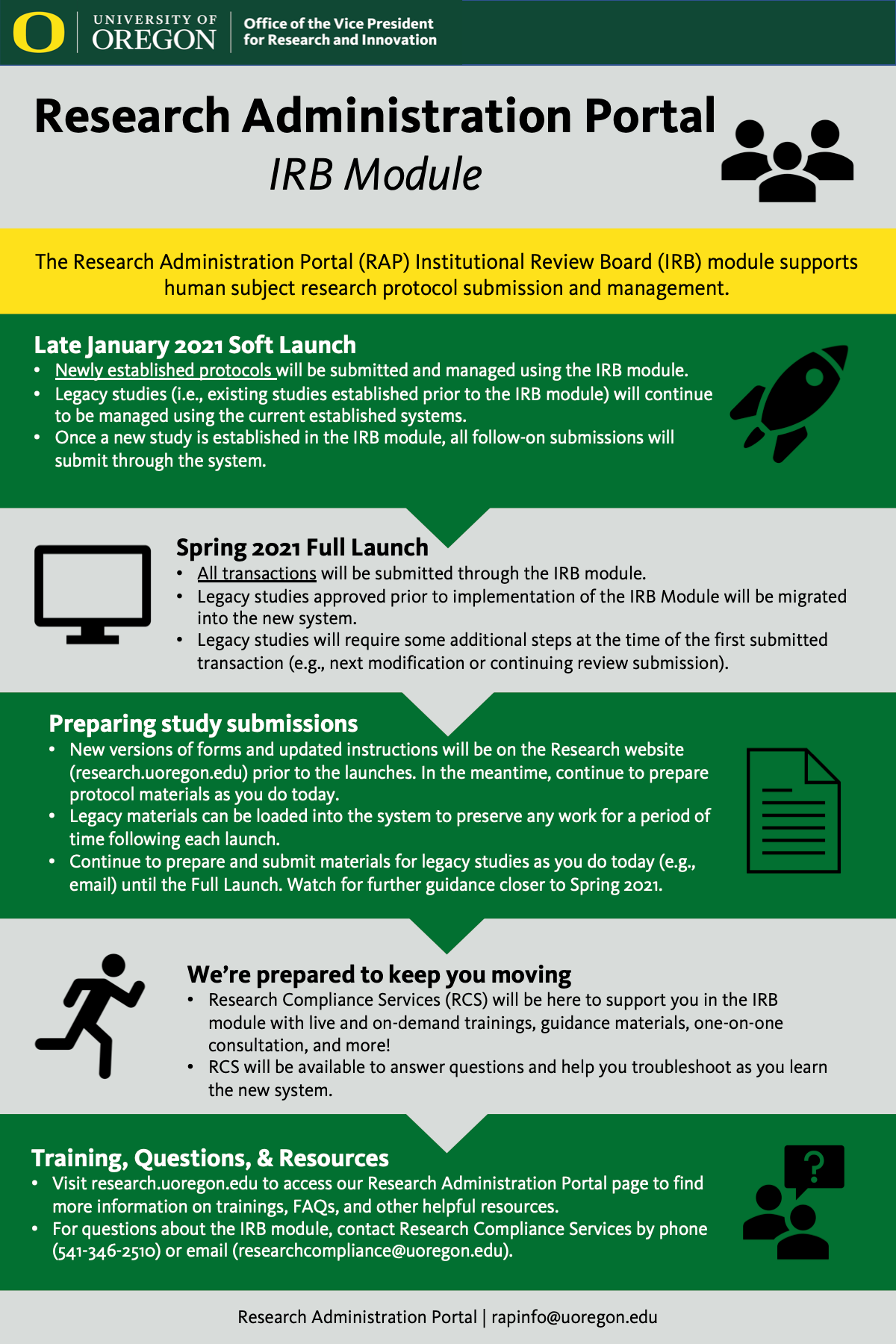
November 2020
During the month of November, the project team accomplished two big milestones:
- System configuration and development completed in early November.
- User acceptance testing completed on November 24
The project team is now shifting their focus for December and January to training and the implementation strategy.
Training
General Information
Research Compliance Services (RCS) will host a series of general information sessions in early January for the research community. These sessions will provide information about the IRB module, what to expect during the transition, and a demonstration of the new system. Information sessions will be recorded and made available on the research website in early January (previously communicated as being available in December).
See our training catalog to find dates and information on registering for the general information sessions.
Training Workshops
Beginning in January 2021, Research Compliance Services will offer several virtual, hands-on training workshops for investigators, research staff, and other stakeholders. During these workshops, investigators and research staff will be able to log into the system, walk through the process of creating a new protocol, and receive support for their upcoming submissions.
See our training catalog for more information on dates, times, and registration links for the first series of training workshops.
Anticipated Go-Live
The project team is planning for a two-stage implementation strategy aimed at ensuring a smooth transition for the community.
- Late January 2021: The first stage will be launched in late January 2021. At that time, newly established protocols will be submitted and managed using the IRB module of the RAP system. Existing studies, including amendments and continuations, will continue to be managed using the current process.
- Spring 2021: The second go-live stage will be launched in Spring 2021. Existing studies will be migrated into the system, after which time all transactions will be submitted through the new system going forward at that time.
October 2020
System configuration and development is nearing the end and is set to complete at the end of October/early November.
The IRB project team has started planning and recruiting users for user acceptance testing (UAT). UAT is set to begin on November 9 and run until November 25.
After UAT is complete, the IRB project team will transition their efforts to training which will run in two phases. The first phase will be in mid-December and the second phase will be in early January. Communications about training will be sent to users ahead of these dates.
For more information and updates about this project, subscribe to the IRB Research Administration Portal email list by visiting the signup page.
September 2020
The system requirements were approved by the VPRI in early September and system development and configuration has started.
Over the next month or two, the IRB project team will be working with the system vendor to develop and configure the new IRB system based on the system requirements and business process changes identified during Onboarding.
To help keep the research community informed about the upcoming changes with the new IRB system, an Around the O story was posted about this project on September 3. Additionally, an opt-in email list has been setup and members of the human subjects research community are encouraged to subscribe. Each month emails sent to this list will highlight process changes that can be expected with the new IRB system. Subscribe to the Research Administration Portal email list by visiting the signup page.
August 2020
System requirements and business process changes were reviewed by the IRB Project Champions on August 18 and will be reviewed by the IRB Module Committee on August 27.
The IRB Project Champions is a group of human subjects research stakeholders from across campus that include researchers, former IRB committee members, and Research Compliance Services staff. The role of the project champions is to:
- Participate in guided tours of the new IRB system
- Provide feedback on proposed changes
- Participate in demonstrations and hands-on exercises
- Advocate for the changes the new IRB system will introduce to the research community
The IRB Module Committee is part of the Research Administration Portal (RAP) governance structure. Similar to the IRB Project Champions group, this committee is made up of human subjects research stakeholders from across campus. All of the IRB Module Committee members are also IRB Project Champions. This committee is responsible for:
- Reviewing and providing feedback on the system requirements scope and change order requests
- Considering impacts on the overall RAP budget
- Providing recommendations to the Program Committee
System configuration and implementation are still scheduled to begin in September after the system requirements are approved by the VPRI.
IRB staff continue to review the HRPP toolkit documents.
July 2020
The IRB project team completed a four-week onboarding process with the vendor to identify system requirements and business process changes required for implementation.
The proposed system requirements and business process changes will be reviewed by the IRB Project Champions and IRB Module Committee (more information about these groups will be shared at a later date). Final system requirements will be approved by the VPRI in August.
Once the system requirements are approved, the vendor will begin an iterative system configuration and implementation process. This is likely to begin in September. Each iteration will be tested by the project team to provide feedback. Stakeholders and end-users may be asked to help with testing.
Additionally, review of the HRPP toolkit will be running in parallel. Over the next few months, starting at the end of July, IRB staff will be doing a more in-depth review of the HRPP toolkit documents and adapt them to UO policies and procedures.
June 2020
The HRPP toolkit has been modified to meet UO’s human subject research needs. The next steps for the toolkit will be a more thorough review of the documents to continue adapting them to UO policies and procedures related to human subject’s research. The IRB staff will begin reviewing documents starting in July.
On Monday, June 8, the IRB system implementation project kicked off. Over the next four weeks, the project team will be reviewing the IRB system and identifying system configurations and/or business process changes that may be required for system implementation.
May 2020
The project team has kicked off the HRPP Toolkit project and development of the toolkit is underway.
The HRPP Toolkit is a standard set of tools made up of standard operating procedures (SOPs), checklists, templates, worksheets, and more. The toolkit will be used as part of the new IRB system. The IRB system implementation project is set to kick off in June.
April 2020
After completing the onboarding process to identify COI system configurations and customizations, it was decided by the project team and governance committees to postpone implementation until the next upgrade of the COI module, scheduled for release in early 2021.
The project team has shifted directions to the implementation of the HRPP Toolkit and the IRB module to support human subjects research. Both of these projects are planned to kick off in May.
March 2020
The COI module implementation is in progress. The RAP onboarding process to identify UO system configurations and customizations is scheduled to be completed in mid-March.
Next Steps:
- Obtain approval on the RAP onboarding configurations and customizations by the COI and Program Module Governance Committees.
January 2020
The project team is currently planning out the program of implementation and beginning to set up governance groups for COI.
